Newsroom
Developing a new analytical technique to assess the quality of blood without breaching the sterility of transfusion bags
Reported by Laleh Solhi from the Brumer lab, Michael Smith Laboratories

Dr. Martha Vardaki
Red blood cell concentrate is the highest-demanded blood product in North American healthcare. Donated red blood cells are an extremely precious resource and there is currently no way to test for blood quality without breaching the sterility of the blood bag. Other than a visual inspection, most available techniques are destructive and require sampling of the bag. In a study recently published in Analyst, Dr. Martha Vardaki, a postdoctoral fellow under Dr. Robin Turner in the Michael Smith Laboratories, in collaboration with the Centre for Blood Research (CBR), developed a new technique to evaluate the quality of transfusion blood without having to compromise the blood bags.
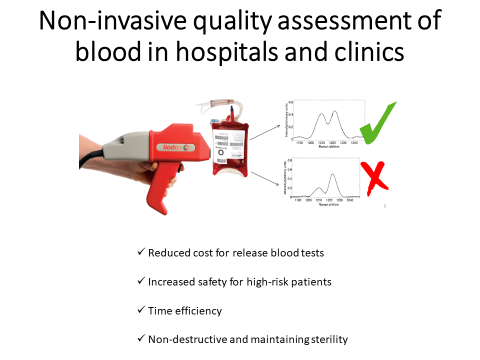
When a donor gives blood, red blood cells are separated from the blood and resuspended in a storage medium. This is then stored in plastic bags in the fridge, where they remain until transfusion. North American regulatory agencies allow a 42-day maximum storage period for bagged red blood cells, and although the cells stay viable during this period, they start to degrade continuously from the first day of storage. A number of chemical and morphological changes result in the accumulation of waste products, oxidative stress, and loss of membrane integrity. This degradation may be responsible for several post-transfusion illnesses. Having a non-invasive quality assessment of blood in hospitals and clinics can increase safety for high-risk patients, reduce total costs of collecting donated blood, and minimize time spent on blood inventory.
 In this study, Martha and her colleagues measured haemoglobin oxygenation of red blood cells in sealed transfusion blood bags consistently using an instrument available on the market. For the first time, they employed a commercially-available spatially offset Raman spectroscopy (SORS) system using a custom-designed protocol to non-invasively explore the biochemical changes in red blood cell concentrate of healthy donors. This experiment was conducted with standard transfusion bags over the standard storage period of approximately 42 days. The results showed an increase in the oxygenation state of haemoglobin over the storage period for all donors, but the change profiles were different for each donor.
In this study, Martha and her colleagues measured haemoglobin oxygenation of red blood cells in sealed transfusion blood bags consistently using an instrument available on the market. For the first time, they employed a commercially-available spatially offset Raman spectroscopy (SORS) system using a custom-designed protocol to non-invasively explore the biochemical changes in red blood cell concentrate of healthy donors. This experiment was conducted with standard transfusion bags over the standard storage period of approximately 42 days. The results showed an increase in the oxygenation state of haemoglobin over the storage period for all donors, but the change profiles were different for each donor.
Dr. Turner’s lab develops instrumentation, advanced signal processing, data analysis, modeling and simulation methods, for biomolecular spectroscopy. His lab applies various forms of vibrational spectroscopy based on Raman scattering to address analytical problems in biochemistry, biotechnology, and biomedical engineering. Dr Turner is also interested in the application of his lab’s technologies to fundamental investigations involving cultured cell systems, tissues, DNA, PNA, LNA, protein-biomaterial interactions, protein-substrate/ligand interactions, and related topics in biotechnology.
Dr. Martha Vardaki and her colleagues in Turner lab at Michael Smith Laboratories in the University of British Columbia developed a new technique to non-invasively measure the quality of transfusion blood during storage.
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada. The full research publication is available in Analyst.
Also featured in:
This research was also highlighted on UBC Applied Science’s Research Spotlights in an article called, “Good blood or bad?“
This research was also highlighted on the Canadian Blood Services’ website in an article called, “New way to check the quality of blood before opening the bag“