Newsroom
Stopping enzymes in their tracks: The synthesis of a novel endo-xyloglucanase inhibitor
Reported by Julie Grondin from the Brumer lab, Michael Smith Laboratories
Complex carbohydrates, or polysaccharides, are the major polymers found in the primary cell wall of all plants. The complete digestion of these complex substrates is orchestrated by a large cohort of carbohydrate-active enzymes, which includes glycoside hydrolases (GHs). Prof. Harry Brumer’s research group at the Michael Smith Laboratories specializes in fundamental and applied carbohydrate enzymology. His group is particularly interested in understanding GHs that are active on a family of plant cell wall polysaccharides called xyloglucans. These polymers comprise a linear β(1-4) glucan (Glc) backbone regularly substituted with xylosyl (Xyl) residues. In many plants, the repeating unit of xyloglucan is a heptasaccharide with the composition Xyl3Glc4, or XXXG.

Namrata Jain, a PhD candidate in the Brumer Lab
Namrata Jain, a PhD candidate in the Department of Chemistry working under Prof. Brumer’s supervision, is designing mechanism-based inhibitors to study endo-xyloglucanase enzymes from the GH5 family. Enzyme inhibitors are routinely used as a tool to characterize enzymes and determine their mode of action. Mechanism-based inhibitors are particularly useful because they act as substrate mimics, and inactivate the enzyme by binding to the active site and inhibiting formation of the product.
In a recent manuscript published in Organic & Biomolecular Chemistry, Namrata describes the synthesis of a mechanism-based inhibitor with a complex XXXG heptasaccharide backbone. The XXXG heptasaccharide is produced enzymatically from tamarind kernel powder, a natural source of xyloglucan. “Mechanism-based inhibitors with small sugar backbones like mono- or disaccharides have been used to study GHs before, but this is the first time that a heptasaccharide has been used as the backbone of such inhibitors,” she noted.
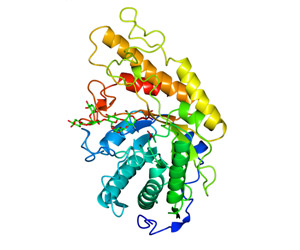
Cellvibrio japonicus GH5 in complex with its mechanism-based inhibitor XXXG(2F)-β-DNP
To synthesize the GH5 endo-xyloglucanase inhibitor, Namrata added two modifications to the XXXG backbone. First, the addition of a chromogenic group to one end of the molecule allows the reaction mechanism cycle to begin as expected. Second, the addition of a carefully positioned fluorine group destabilizes the reaction, effectively stopping the enzyme in its tracks. “One of the challenges of this synthesis is that traditionally, adding the fluorine and chromogenic groups results in a mixture of four different stereoisomers,” Namrata said. In cases where the inhibitor backbone is a simple mono- or disaccharide, resolving this mixture of stereoisomers and isolating the desired product is possible by chromatography. However, this kind of resolution was not possible in this case due to the size of the heptasaccharide backbone, meaning that Namrata had to optimize the synthesis itself in order to obtain the desired final product.
Next, Namrata confirmed that this inhibitor was active against three different GH5 endo-xyloglucanases. In collaboration with Prof. Gideon Davies from the University of York in the UK, they were able to solve the crystal structure of a GH5 enzyme from a soil bacterium called Cellvibrio japonicus in a covalent complex with the inhibitor. “We were able to freeze the enzyme during its catalytic cycle, and because the backbone of the inhibitor is a substrate mimic, we now have very detailed information about the interaction between the enzyme and the substrate.”
The successful use of this inhibitor in studying endo-xyloglucanases from GH5 will serve as a platform for the study of enzymes from a variety of sources. “With this one molecule, we can study a range of enzymes with a similar mechanism and sugar specificity.” Namrata’s next goal will be to extend this established protocol to design inhibitors for enzymes active on a range of oligosaccharides. Overall, understanding the molecular mechanisms of enzymatic carbohydrate degradation has direct applications in the field of plant biomass conversion for biofuels and high-value bioproducts, fibre materials engineering, and enzyme engineering.
Read the full study in Organic & Biomolecular Chemistry.