Newsroom
The Future of Blood Transfusion and Organ Transplantation: A Universal Blood Story
Writing and diagram by Deepesh Panwar from the Brumer Lab, Michael Smith Laboratories

Prof. Stephen Withers
Blood transfusion and organ transplantation are cornerstones of modern medicine, saving millions of lives each year. However, the persistent challenge of matching donor and recipient blood groups often hampers their widespread application. Incompatibility within the ABO blood group system—particularly involving A, B, and H (O-type) carbohydrate antigens—can trigger severe immune reactions, leading to rejection or other life-threatening complications.
For instance, if a donor with A-type antigens donates blood or an organ to a recipient with O-type blood, the recipient’s immune system recognizes the A antigens as ‘foreign’. This leads to the immune system aggressively targeting foreign antigens, which can destroy donor cells and lead to possible rejection or complications.
To address this critical issue, Professor Emeritus Stephen Withers (Department of Chemistry, Associate Member, Michael Smith Laboratories) and Professor Jayachandran Kizhakkedathu (Department of Pathology and Laboratory Medicine, Centre for Blood Research) have co-developed a transformative technology. Their enzyme-based platform can convert A antigens in the blood and organs into the universal H (O-type) antigen.
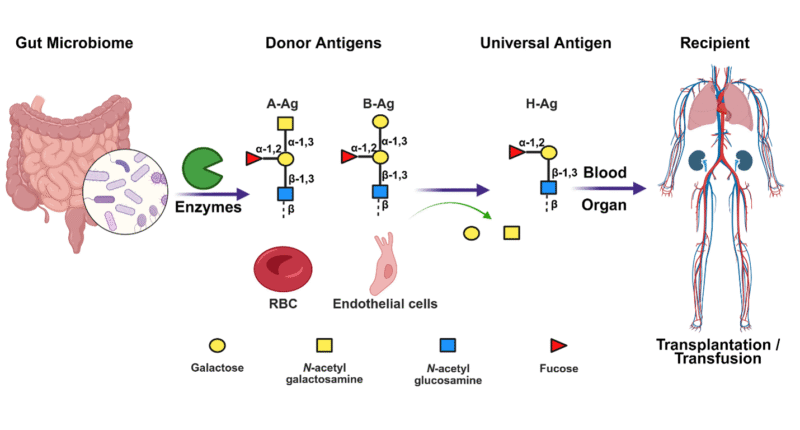
Mining human gut microbial enzymes for A or B to O conversion and their application in blood transfusion and organ transplantation (Created with BioRender.com)
In a recent conversation, Prof. Withers shared insights into the evolution of this breakthrough, its implications, and the road ahead.
How has this project evolved since its inception, and were there any major challenges your team faced?
Our group, in collaboration with Prof. J. Kizhakkedathu, began working nearly a decade ago to identify enzymes capable of converting A and B blood group antigens into the universal H (O) antigen. Initially, we tested engineered enzymes created in our lab and compared them with various enzymes reported in the literature for their ability to cleave blood group glycan antigens. Unfortunately, however, these enzymes were either not efficient or specific enough, or did not work well on intact blood cells.
To overcome these limitations, we shifted our attention to the human gut microbiome—which is a collection of genes present in gut microbes. Leveraging our experience in the use of metagenomic approaches to screen enzymes from the gut microbiota, we successfully discovered a new class of enzymes capable of converting A antigens to the O-type phenotype, work that was published in Nature Microbiology. One of the most exciting discoveries was that these enzymes are remarkably potent, requiring only microgram-level quantities of the enzyme to treat entire organs—dramatically lower than earlier estimates requiring gram-scale doses. This greatly reduces the risk of systemic side effects and makes large-scale application more feasible.
I found the idea of mining enzymes from gut microbiota fascinating as your work showed efficient conversion of A to O antigens even in organs. Did you consider exploring other environments for potential enzymes?
The human gastrointestinal tract naturally expresses a diverse array of glycans, including A and B antigens, which made it an ideal environment for our enzyme hunt. The human gut microbiome has evolved to interact with these glycans, increasing the likelihood of identifying enzymes that met our criteria.
While we considered other environments, such as leech or mosquito guts, which feed on human blood, those environments are of limited use due to their practical feasibility, so we focused on human gut.
 Has your lab found enzymes capable of B to O conversion as well?
Has your lab found enzymes capable of B to O conversion as well?
Building on our success with A to O conversion, we’ve also mined enzymes for B to O conversion from the human gut. Although these efforts are still ongoing, our initial results are promising. Our ultimate goal is to create an enzyme cocktail capable of converting any ABO blood type to the universal O antigen, to eliminate blood type incompatibility altogether.
Your findings mention the re-emergence of antigens on organ surfaces. How might this challenge be addressed moving forward?
During pre-transplantation procedures, organs are perfused outside the body, meaning they are washed with a solution containing our enzymes which remove the blood antigens. Our treatment can convert 80–97% of A antigens to H-type within a few hours. However, the antigens will reappear post-transplantation.
We had imagined that this would be a huge barrier to our method being implemented, however, transplant specialists were able to tell us that our pre-treatment process was still beneficial as a way to reduce rejection risk. A key factor here is what we call “accommodation” of these carbohydrate antigens—where the recipient’s immune system gradually adapts to the donor organ, as the antigens re-emerge. We are, though, still actively looking into this with our collaborators to understand more about re-emergence kinetics.
How does your enzyme-based approach compare to other strategies used in transplantation to reduce rejection risks, and which organs are you currently targeting in your work?
ABO-incompatible transplantation is sometimes done, especially in kidney transplants in cases where there is a low density of antigens present on the organ. In those cases, clinicians can pre-treat the recipient with plasmapheresis, a process that removes antibodies from the bloodstream, before administering immunosuppressant medication to manage rejection risks by suppressing the immune response. However, these protocols are limited to live donor situations and carry their own risks.
Our approach offers a safer and more direct alternative—removing the primary cause of rejection at its source. Currently, our focus is on kidneys and lungs. Kidneys are a high-demand organ with relatively low risk, making them an ideal target. Lungs, on the other hand, benefit from highly advanced perfusion technologies. In fact, our collaboration with a Toronto-based lung transplant surgeon led to a successful proof-of-concept study and our first major publication in Science Translational Medicine, which paves the way for global partnerships in this area.
What are the main challenges ahead in commercializing these enzymes and implementing them in clinical settings?
There are multiple hurdles on the path to commercialization, but I think the largest one is funding. We currently run a small team with limited resources. To progress to human clinical trials, we need to meet rigorous Good Manufacturing Practice (GMP) standards, which requires substantial investment. As we continue our preclinical work, we are actively seeking funding from venture capitalists and industry partners. The growing global interest in organ transplantation and universal blood compatibility makes us optimistic about future support and broader adoption.
The journey toward universal blood and organ compatibility is no longer a distant dream. Through pioneering enzyme technology, Prof. Withers, Prof. Kizhakkedathu, and their collaborators are charting a new course in transfusion and transplantation science. By removing blood-type barriers, this innovation has the potential to significantly expand the donor pool, reduce waiting times, and save countless lives around the world.
As research continues and clinical validation progresses, the universal blood story is fast becoming a defining chapter in the future of regenerative medicine.
Quick links:
- Read the team’s publication in Nature Communications on A to O conversion in kidneys
- Read the team’s publication in Nature Biomedical Engineering on rejection prevention in the lungs
- Read their review in the Journal of Biological Chemistry about universal donor blood