Education and outreach
Celebrating Gairdner week with science comics
In celebration of Gairdner week, the Michael Smith Laboratories at UBC, the Canadian Society for Molecular Biosciences, and the Gairdner Foundation have collaborated to create a set of graphical resources aimed at middle/high-school students and the general public. These resources celebrate the science of this year’s Gairdner Award winners and are designed to explain the research behind each Gairdner Award and to emphasize the value of fundamental discovery-based research to society.
Contents
- Plants: Amazing Phyto-Pharmacies
- Kinesin: The Little Engine that Could
- DNA Replication: Not your Office Photocopier
- Integrins and the Social Network of Cells
- Discussion Questions
5.1. For all four articles:
5.2 For “Kinesin: The Little Engine that Could”:
5.3 For “DNA Replication: Not your Office Photocopier”:
5.4 For “Plants: Amazing Phyto-Pharmacies”:
5.5 For “Integrins and the Social Network of Cells”: - Download printable documents
Plants: Amazing Phyto-Pharmacies
Research by Dr. Susan Band Horwitz – 2019 Gairdner award winner
Written by Alison McAfee
Art by Armin Mortazavi
October 2019
Some of the best chemical engineers in the world don’t have a university degree. The most prolific institutions (if they can be called that) don’t have professors or laboratories. The chemicals themselves boast more complicated architectures than humans could imagine. The working conditions are dirty and crowded, but somehow the immobile chemists still succeed. These thriving chemical production plants, in every sense of the word, are forests.
 Some phytochemicals (compounds synthesized by plants) that nature has so elegantly engineered are also life-saving medicines. Take Taxol, a compound originally isolated from the bark of Pacific yew trees. Almost sixty years ago, when Arthur Barclay, a botanist, collected yew bark from a Pacific Northwest forest, he had no idea that one of the phytochemicals the bark contained would save patients’ lives. Two years later, Drs. Mansukh Wani and Monroe Wall discovered a potent anticancer compound in the yew bark sample, which they named Taxol (later trademarked, and also known as paclitaxel today). They soon realized that Taxol’s molecular structure was extraordinarily complex – more elaborate than a human chemist could have ever conceived. But it was Dr. Susan Band Horwitz, a distinguished professor at the Albert Einstein College of Medicine, New York, who made Taxol famous.
Some phytochemicals (compounds synthesized by plants) that nature has so elegantly engineered are also life-saving medicines. Take Taxol, a compound originally isolated from the bark of Pacific yew trees. Almost sixty years ago, when Arthur Barclay, a botanist, collected yew bark from a Pacific Northwest forest, he had no idea that one of the phytochemicals the bark contained would save patients’ lives. Two years later, Drs. Mansukh Wani and Monroe Wall discovered a potent anticancer compound in the yew bark sample, which they named Taxol (later trademarked, and also known as paclitaxel today). They soon realized that Taxol’s molecular structure was extraordinarily complex – more elaborate than a human chemist could have ever conceived. But it was Dr. Susan Band Horwitz, a distinguished professor at the Albert Einstein College of Medicine, New York, who made Taxol famous.
“The structure was very intriguing and beautiful,” says Horwitz, who recently received a Gairdner Award for her research on Taxol. “All my career, I’ve been interested in trying to understand how small molecules from natural products could be used in the treatment of cancer.” And years after Wani and Wall published Taxol’s structure and anticancer properties, the researchers still weren’t sure how it worked. Not a single paper had been published on it. “Nobody had any idea how [Taxol] killed the cells. To move a drug forward, it’s very important to understand what it does in an organism.”
It didn’t take long for Horwitz and her student to realize they had something special. “I had a student looking for a thesis project, and I said to him, ‘Let’s get ten milligrams [of Taxol] and you can work on it until the end of the month,’” Horwitz recalls. “That end of the month never arrived, because I am still doing experiments on the drug!” They knew they had something really exciting when they saw that it killed cells in a way that no one had seen before. As Horwitz says, that kind of discovery is “what science is all about.”
Horwitz and her graduate student at the time, Peter Schiff, figured that Taxol killed cancer cells in a new and unusual way, which meant that it could save lives where other drugs failed. Cancerous tumors are masses of cells multiplying out of control, so anticancer drugs need to preferentially kill fast-growing cells. Most other drugs work by damaging cells’ DNA or disrupting DNA synthesis, both of which put the brakes on cell division and therefore abolish tumors (or at least slow them down). The trouble comes when the drugs stop working, or if they never work in the first place. Just like fast-growing bacteria can acquire resistance to antibiotics, cancerous cells can acquire resistance to chemotherapy, and when they do, it’s critical for doctors to have a different weapon up their sleeve to increase the odds of finding one that works.
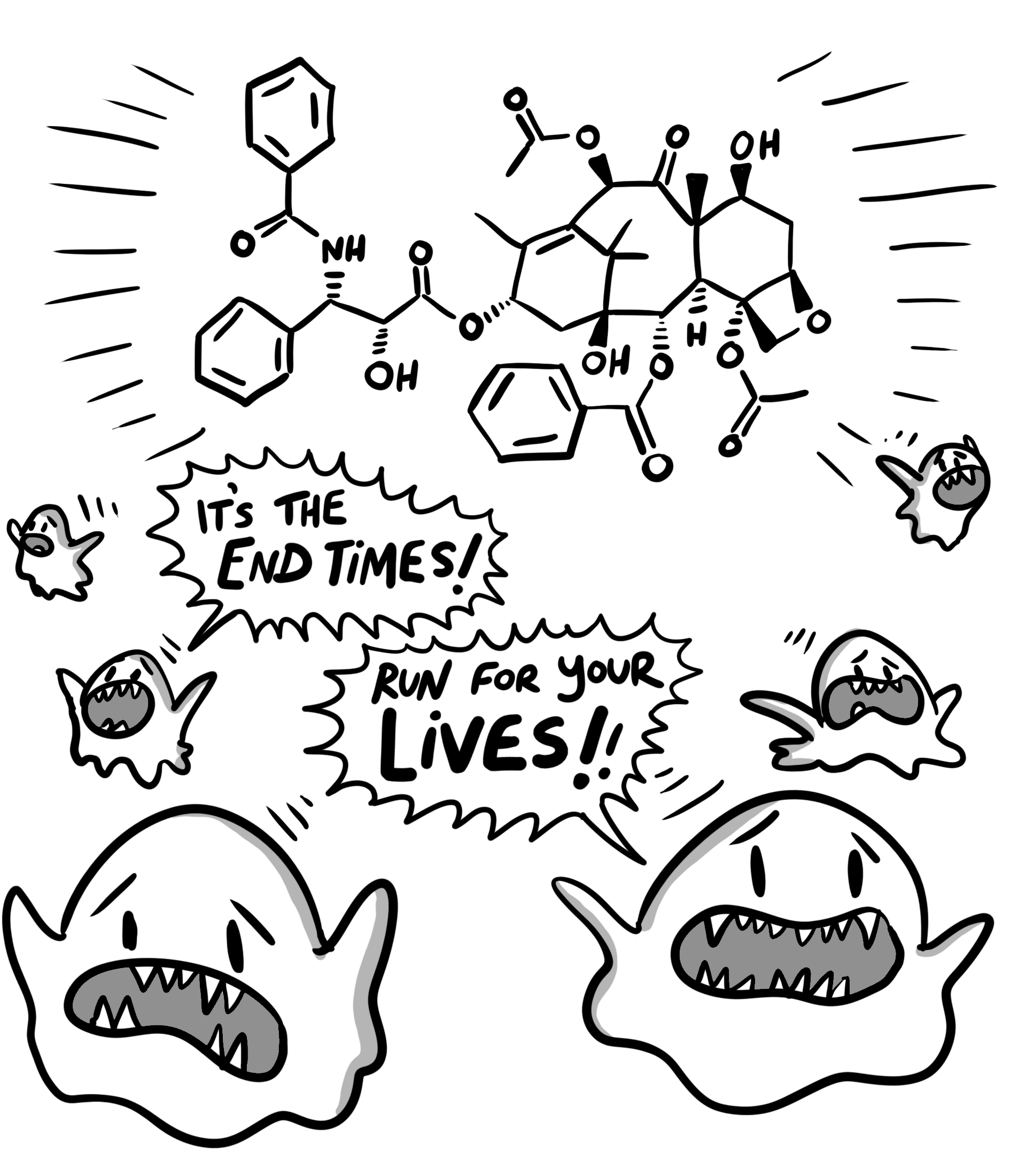
Schiff and Horwitz found that unlike most other chemotherapies, Taxol binds to the cell’s microtubules (protein filaments that help move things around inside the cell). Those microtubules are vital for cell division – they lengthen and shorten to drag the chromosomes to opposite ends of the cell before it splits, much like a microscopic tug-of-war. When Taxol binds to the microtubules, the tugging stops – the filaments bundle up and seize, the cells can’t divide, and the tumor stops growing. Targeting the microtubules is an entirely different strategy than targeting DNA, adding a whole new weapon to doctors’ arsenal. At the time, this was a totally novel mechanism – today, microtubule-targeting agents form a whole class of chemotherapies, and Taxol is the most widely used of all. It has extended millions of patients’ lives and generated billions of dollars in revenue. All because of the humble Pacific yew.
Plants and their elaborate phytochemicals are invaluable reservoirs for anticancer drugs. Cancer is responsible for nearly one third of all deaths in Canada, making it the frontrunning cause of death nation-wide. It is so deadly in part because it is a notoriously complex disease, and this complexity means that designing or identifying drugs that will be effective for more than a tiny subset of patients is extremely difficult. Worse yet, the disease is caused by out-of-control proliferation of our very own cells, not a pathogen or toxin, so any anti-cancer drug is inevitably toxic to at least some of our healthy cells, too. The ideal chemotherapy would wipe out cancer cells but leave healthy cells unharmed (or at least, less harmed), but finding such a chemical is like looking for a needle in a forest. No – it’s like looking for a needle in a forest, when we don’t even know what a needle looks like.
No one thought that the yew bark sample Barclay collected would yield a blockbuster drug. No one even knew that it was a needle. The sample was randomly chosen to be part of a massive anticancer screening program spearheaded by the National Cancer Institute, and was one of 30,000 samples analyzed under the program. But other plants, like the American mayapple – a woodland plant found in Eastern US and Canada – and the rosy periwinkle – a flowering plant endemic to Madagascar – among others, had yielded similarly powerful anticancer drugs (podophyllotoxin, vincristine, and vinblastine, also known as antimitotic agents) in the past, but we still don’t fully understand why they exist.
 Horwitz grew up in a small seaside Massachusetts town. She went to college intending to major in history, but after taking a required biology course, she changed her path. Not satisfied with the kinds of jobs she could get with a Bachelor’s degree in biology, she obtained a PhD in biochemistry at a time when very few women entered academia. “Once, I asked a faculty member where the ladies’ room was after an interview,” Horwitz reflects. “The professor looked at me and said ‘Hmm . . . I think there’s one in the basement?’” She settled on studying at Brandeis University because, to her delight, there were two female faculty members in their biochemistry department. Horwitz is a recipient of the 2019 Canada International Gairdner Award, which recognizes the best biomedical scientists in the world.
Horwitz grew up in a small seaside Massachusetts town. She went to college intending to major in history, but after taking a required biology course, she changed her path. Not satisfied with the kinds of jobs she could get with a Bachelor’s degree in biology, she obtained a PhD in biochemistry at a time when very few women entered academia. “Once, I asked a faculty member where the ladies’ room was after an interview,” Horwitz reflects. “The professor looked at me and said ‘Hmm . . . I think there’s one in the basement?’” She settled on studying at Brandeis University because, to her delight, there were two female faculty members in their biochemistry department. Horwitz is a recipient of the 2019 Canada International Gairdner Award, which recognizes the best biomedical scientists in the world.
Plants don’t get cancer the way humans do, so the exact reason why plants produce antimitotic agents in the first place is puzzling. Scientists think it could be a form of chemical defense against herbivores: Plants can’t run away from leaf-munchers, so instead they stand their ground and make sure their attacker gets sick and doesn’t come back. Animals (including people) become ill when they eat the leaves and bark with toxic compounds because the antimitotic agents harm fast-growing, healthy cells too (like those in the stomach lining) – not just tumors. In fact, that’s part of the reason why the chemotherapy comes with debilitating side effects. But when the alternative is death by cancer, most people would still choose chemotherapy.
Plants, with their extraordinarily diverse phytochemicals, have given us far more than just chemotherapies. Yes, more than just illicit drugs, like opium, cocaine, and ecstasy, too. Around fifty percent of all prescription drugs in North America are derived from or inspired by chemicals found in nature, and plants are major contributors. The Barbasco root (a type of Mexican yam) gave us the birth control pill. The willow tree gave us aspirin. The quinine tree gave us antimalarials. Fungi, too, have given us famous drugs like penicillin and Lipitor. All of these drugs, including our beloved caffeine, have profoundly changed the world.
And there are probably many more plant-based medicines that we don’t yet know about. We haven’t even discovered all the plant species that exist – around 2,000 new plants are described every year – let alone tested all their phytochemicals for medicinal properties. There are likely countless other antimitotic agents and other drugs that we don’t know about. But sadly, our precious forests and wild plants are vanishing.

Habitat destruction – slash and burn deforestation for agriculture, clear-cut logging, toxic waste from mining, and drilling for fossil fuels, to name a few – is threatening global biodiversity, including plants. Unprecedented forest fires, like those burning the Amazon rainforest – a hotbed of plant species richness – and boreal forests in Canada, Alaska, and Siberia, are eliminating tens of millions of acres of forests in one fell swoop. With so much habitat destruction, some species are failing to persist. The rosy periwinkle (which gave us vincristine and vinblastine, two of the first plant-based anticancer drugs) for example, is endangered in its natural range due to habitat destruction and heavy slash and burn deforestation. Luckily, the cute flower is also a popular ornamental, so has been naturalized in other habitatslocations. But other species are not so lucky.
Hundreds, possibly thousands of plant species have been wiped out since the 1700s. Scientists have documented at least 572 extinct plants, and most agree that it is likely a gross underestimate. And according to a recent UN report, 1 million animal and plant species are threatened by extinction. With this trajectory, many species will be wiped out before we can even investigate their medicinal value. And even if we discover new drugs in time, we still need to preserve the environment to ensure our future supply.
That’s because some phytochemicals are so complex, we still can’t synthesize them without the plant’s help – even if we already know the structure. Today, there are still only three ways to produce Taxol, and all depend at least partially on harvesting raw plant material. Taxol can be extracted directly from yew bark, just like Wani and Wall did in the 1960s. But this process kills the slow-growing tree, and the global Taxol demand is too high (according to some estimates, bark from eight 60-year-old trees would be needed to treat just one patient). Today, Taxol is commercially produced either by extracting it from yew cells cultured in the laboratory or by a complex process involving an extraction from yew needles (leaves) coupled with laboratory reactions. The latter method, though it’s much more efficient than bark extraction and doesn’t kill the tree, still requires harvesting massive amounts of needles.
“Most people don’t realize how many of our drugs come from natural products,” says Horwitz. But we are destroying the Amazon, we are destroying the trees, and we are destroying our chances of discovering new life-saving, phytochemical medicines. Horwitz urges us that appreciating, respecting, and preserving nature is very important. “No chemist would ever sit down and design a drug like Taxol, and yet the tree does it. And the tree gives the chemist ideas.”
Learn more about UBC’s Michael Smith Laboratories, the Gairdner Foundation, and the Canadian Society for Molecular Biosciences.
Kinesin: The Little Engine that Could
Written by Shawn P. Shortill
Art by Armin Mortazavi
October 2019
When we are learning about cells and how they function, we are often confronted with large, circular blob-like things that contain several different internal structures for us to memorize. It may be tempting to dismissively think of cells as being nothing more than these simple, two dimensional objects (after all, they are commonly referred to as “the most basic units of life”). However, closer inspection reveals that cells are extremely dynamic, and their daily activities are far more exciting and complicated than first appearances may suggest. Cells operate like tiny machines and their inner workings are highly coordinated and exquisitely complex. Each component of a cell must be carefully maintained and precisely localized in relation to the other components for cells to function appropriately. When all of these seemingly isolated components and processes are synchronized correctly, they cooperate to create life.
To begin understanding the complexity of a cell, a helpful comparison can be made between cells and busy cities. The different districts of a city, each with its own important task such as food or energy production, can be thought of as the various functionally distinct organelles within a cell. A power plant, for example, converts raw materials into usable energy for the city much like how the mitochondria of a cell generates chemical energy from environmental nutrients. However, a power plant in the industrial district can only produce energy when skilled engineers and workers are present, true also for the cell which needs specialized organelle-specific proteins to be at the right place at the right time in order to perform their work.
To effectively travel about this hypothetical city the citizens and workers require motor vehicles for transportation, this too parallels strategies employed by cells. While a car would travel along a highway to reach its destination, specialized proteins involved in cellular transport travel along rigid biological tracks such as microtubules. Microtubules are part of a family of scaffold proteins which collectively form the cytoskeleton, a filamentous support network which helps maintain the shape of the cell and prevents it from collapsing.

The proteins that travel the cytoskeleton are known as motor proteins and they are the nanoscopic machines required to turn the cells chemical energy into active motion. They provide a means to traffic molecular cargo, such as other proteins or entire organelles, from one intracellular destination to another allowing the cell to grow and adapt. One important class of molecular motors are the kinesins and their discovery marked one of the most significant milestones in furthering our understanding of the inner workings of the cell.
Working with the renowned molecular biologist Dr. Michael Sheetz, a young scientist named Ronald Vale made a discovery that not only changed the way scientists think about cells, it also laid the foundation for an entirely new field of science; mechanobiology or the study of how physical forces are involved in biological processes. This exciting research began with a 1985 publication in the prestigious scientific journal Cell, where the authors demonstrated the presence of a protein that associates with cytoskeletal microtubules and facilitates the movement of organelles along their length. Ronald and his colleagues decided to investigate the basis of microscopic motorized transport using neurons, the signal-transmitting cells of our nervous system. Neurons are a type of cell that rely particularly heavily on intracellular transport to send their messages in a timely manner due to the fact that they are far longer than most cells. The logic behind their decision to study in neurons was clever; the longer the highway, the more necessary motorized vehicles must be to travel along it.
Vale and his team began their investigations by harvesting an axon, the very long, slender portion of a neuron, from a giant squid and performing biochemical tests on it to identify proteins involved in motor transport. The choice to use the axon of a giant squid was instrumental to their discovery as it was easier to harvest and manipulate than that of other organisms due in part to its extraordinary size. Their efforts eventually paid off, as Ronald Vale and his colleagues soon identified a new protein which they showed plays an important role in cellular motor transport. Once the movement-promoting properties of their new protein were reported, they proposed the name kinesin from the Greek term kinein, meaning “to move”.
Following Ronald’s initial report in 1985, his research group and others worked diligently to establish a variety of important roles for kinesin in biological processes ranging from nervous system signaling to cell division. More recent insights from work in model systems like budding yeasts, nematode worms and fruit flies have shed light onto the molecular mechanisms of kinesin structure and function allowing unprecedented investigation of this motor protein.

Structurally, kinesins are comprised of two regions; a stalk region which ends in a long, flexible cargo-binding tail and a head region, containing the functional motor. The head region binds to microtubules and facilitates movement along their length in consecutive steps. This molecular motion doesn’t come cheap however, as the cell must pay for it with chemical energy stored in the form of ATP, the cells energetic currency. The head region of a kinesin molecule moves along by binding and destroying ATP, causing a change in the organization of the head group which ultimately results in the entire kinesin pulling itself forward on the microtubule. For every step that a kinesin takes, one molecule of ATP must be spent. This means that a cell must carefully regulate how and when it uses its kinesin transport system as it is energetically quite costly, and many other important cellular processes also require the use of ATP.
Meanwhile at the other end of the kinesin, the tip of the tail region is recognizing and tightly binding to cargo that needs to be transported to a new location. By working together, the head and tail regions of the kinesin molecule allow very large structures such as mitochondria to be efficiently moved around the cell to their final destinations. These mechanistic insights into the function of kinesin have advanced our understanding of cell biology immeasurably and highlighted its ability to influence a variety of biological processes.
The discovery of kinesin not only furthers our understanding of basic biology but provides new insights into the pathology of human diseases. These diseases include various forms of cancer, Alzheimer’s disease, a neurological disease called Charcot-Marie-Tooth disease Type 2A (CMT2A) and polycystic kidney disease. The complex nature of cellular processes such as kinesin-mediated movement makes teasing apart the different contributing factors of complex diseases like these challenging. By better understanding the molecular mechanisms of these neurological diseases and other kinesin-related diseases, treatments may begin to be developed resulting in improved quality of life or even a complete curing of disease symptoms.
Undoubtedly, Ronald Vale’s exciting discovery in the mid-1980’s marked a major event in the history of cellular studies. Providing pivotal insights into fundamental biology and disease pathology alike, his pioneering work has created many exciting opportunities for continued investigation into the inner workings of the cell. Ronald Vale is a 2019 recipient of the Canada Gairdner Award, a prestigious honour that recognizes biomedical scientists behind the most exciting and impactful research every year. The work of visionary scientists like Ronald Vale allows us to constantly push the boundaries of what we imagine to be possible, and the discovery and continued characterization of the molecular motor kinesin certainly has cellular science moving in an exciting direction.
Learn more about UBC’s Michael Smith Laboratories, the Gairdner Foundation, and the Canadian Society for Molecular Biosciences.
DNA Replication: Not your Office Photocopier
Written by Krysta Coyle
Art by Armin Mortazavi
October 2019
If the letters in the human genome, your DNA code, were a book, it would take a person typing 60 words per minute, 8 hours a day, around 50 years to copy the 3 billion base pairs. This incredible task of replicating all the genetic material in a cell must be accurate, must be copied only once, and must be complete at a specific point in time. The smallest mistakes can be disastrous. Sounds impossible? Not so fast – cells in your body are dividing as you read, and here’s the incredible part: copies of your DNA are made in hours – not months or years.
DNA replication, the copying of all genetic material in a cell, is an essential process for life. As cells divide to support growth, heal wounds, or replace damaged cells, the genetic instruction manual – the genome – must be reproduced efficiently and correctly. With 1.8 metres of DNA coiled tightly into the nucleus of every cell, this is not as straightforward as pressing ‘copy’ on your office photocopier. The efficiency of DNA replication in humans and other multi-cellular organisms relies on replication starting at multiple different sites, called origins of replication, throughout the genome. This is an elegantly choreographed process, not unlike watching a photocopier collate multiple documents simultaneously. It relies on numerous proteins and DNA molecules interacting at exactly the right time in exactly the right location to make an exact copy of the genome.
Forty years ago, Dr. Bruce Stillman joined the Cold Spring Harbor Laboratory as a postdoctoral fellow investigating DNA replication. He reminisces about colleagues thinking, “we knew everything about DNA replication [in 1979].” Both Stillman and Dr. John Diffley, a postdoctoral fellow who worked with Stillman in the 1980s, have since spent their scientific careers uncovering the machinery that makes precise DNA replication possible in such a short time.
“Unless [replication] started at many different places, it would take months to duplicate the entire genome. But it only takes about 8 hours,” says Stillman, President of the Cold Spring Harbor Laboratory. Stillman and Diffley recently received the Gairdner Award for their work on DNA replication. When cells are preparing to divide, multiple proteins assemble at thousands of unique starting points. This set of proteins is known as the origin recognition complex, first discovered in yeast by Stillman, the discovery of which set the stage for further investigation of many additional proteins and molecules involved in initiation and control of DNA replication.
Controlling the process of DNA replication – ensuring that no part of the genome is replicated more than once – is critical. If thousands of people started photocopying an encyclopedia, each starting at a different point, surely they would make a mistake: they would copy some sections more than once, and other sections might not be copied at all. Unlike an extra page or a missing line in a book or newspaper, confusion at the cellular level can be catastrophic. Simple errors, such as small regions of the DNA copied more than once, or sections that aren’t copied at all, can have terrible results.
Astonishingly, the cell gets it right most of the time. Diffley was challenged to understand how these multiple starting points could be used once in every cycle of DNA replication – but never more than once. This level of precision and accuracy is accomplished by tightly linking the timing of DNA replication to the steps of cell division, known as the cell cycle.
When a cell is preparing to divide, the proteins that make up the origin recognition complex are recruited to the thousands of starting points in the genome. This is only possible when a cell is ready to divide, through the actions of proteins called kinases. Kinases prevent the assembly of the complex before cell division. Once the complex is assembled, it is joined by additional proteins, including a helicase, which is required to unwind the tightly coiled DNA. All of these proteins together indicate to the cell that the site is ready to be used for DNA replication. A full roster of proteins triggers the unwinding of the DNA, recruits the enzyme responsible for generating a copy of the DNA (DNA polymerase), and replication begins.
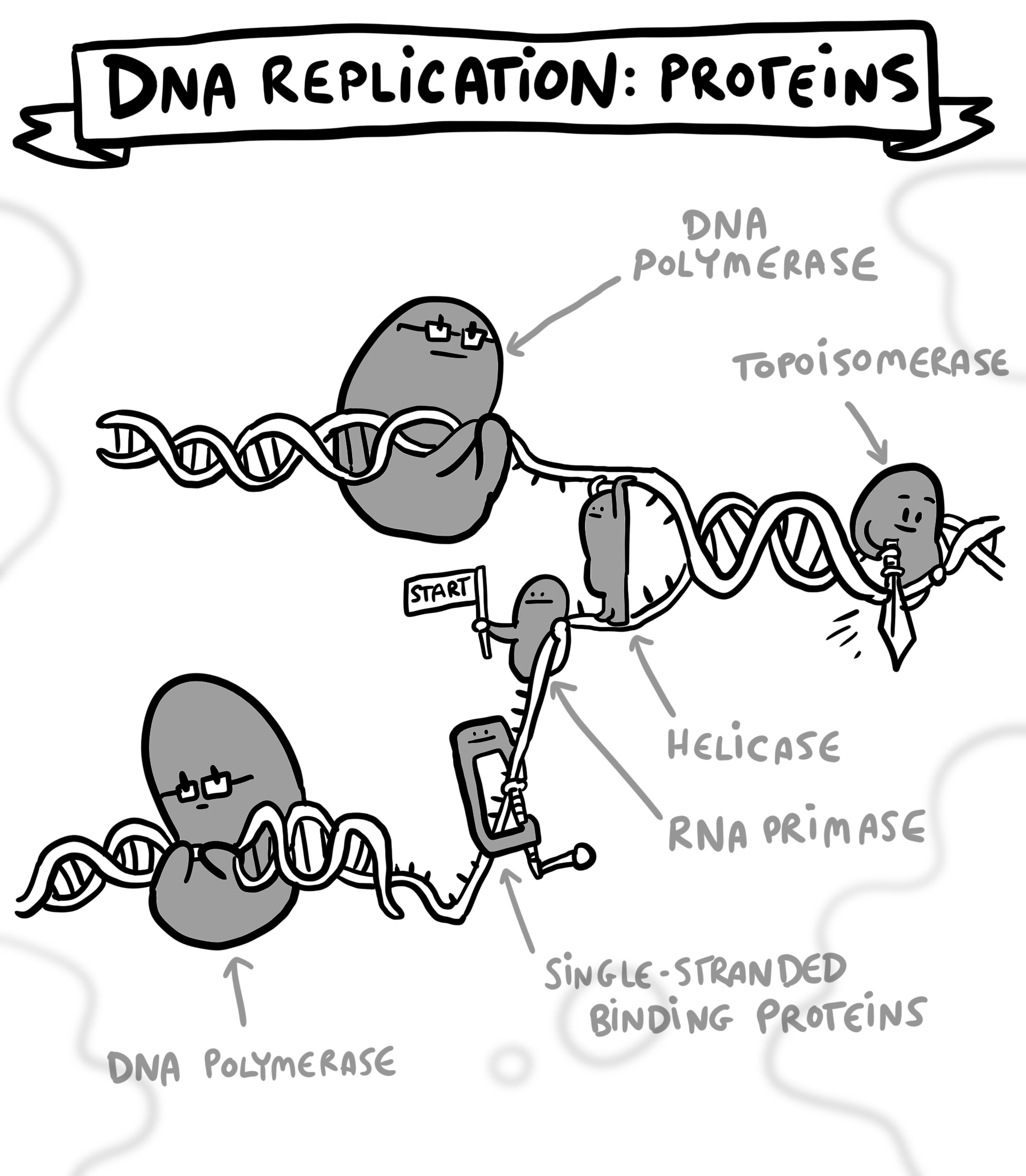
Proper DNA replication is one of the most critical aspects of cell division and understanding the process can help physicians and scientists understand various aspects of human health. Dysregulation of replication can be disastrous. Simple errors during the process of replication, resulting in incorrect amounts of gene products, may cause uncontrolled cell division resulting in cancer. There are some features of cancer that contribute to these kinds of errors, such as mistakes in how cells divide, but cancer progression itself can cause increased errors in DNA replication. A higher-than-normal rate of mutations in cancer cells can result in an accumulation of mutations and complex gene rearrangements. These mutations often make cells aggressive, resulting in tumor formation. Genomic instability also causes large variation between cancer cells, which makes it harder to identify and kill every cancer cell in a tumor.
Paradoxically, while cancer cells can become more aggressive through mutations, or errors in DNA replication, this process is also a compelling target for anti-cancer therapies. Many current chemotherapies interfere with the process of DNA replication. By targeting a process which is essential to all replicating cells in the body, these anti-cancer agents often have toxic side effects. As Diffley shares, “the future is to identify more precise treatments that aren’t as toxic.”
Some mistakes in DNA replication are heritable, caused by mutations found in many cells of the body. One such defect is known as Meier-Gorlin syndrome, a condition which is characterized by small stature. This is considered a form of primordial dwarfism as growth problems begin before birth. Mutations in components of the origin recognition complex are associated with this syndrome, and scientists suspect that the mutations impair the formation of the origin recognition complex. This results in delayed cell division, which impairs tissue and bone growth during development.
Scientists like Stillman and Diffley are still working to characterize DNA replication from start to finish, and we are only beginning to understand the complexities of the process. Although Stillman’s colleagues thought we knew everything there was to know about DNA replication 40 years ago, there are still many aspects left to uncover and understand. Just think – if Stillman and Diffley had started typing out the genome in the early 1980s, they’d still be at it now.
Learn more about UBC’s Michael Smith Laboratories, the Gairdner Foundation, and the Canadian Society for Molecular Biosciences.
Integrins and the Social Network of Cells
Written by Daniela Salas Acosta
Art by Armin Mortazavi
October 2019
When imagining a cell, it’s often easy to think of it as a lone entity, a single squishy bubble of fluid. However, cells are actually incredibly complex, and they can organize in ways to form many types of structures and ultimately whole living organisms like a tree, a bird, or even our own bodies! Understanding how these cells interact and get together is key to understanding how different cells make different structures – like how bones are different from skin for instance. Knowing this also reveals how cells talk to one another. And just as we like to hug some people but prefer to only text others, the cells in our bodies have different ways of interacting.
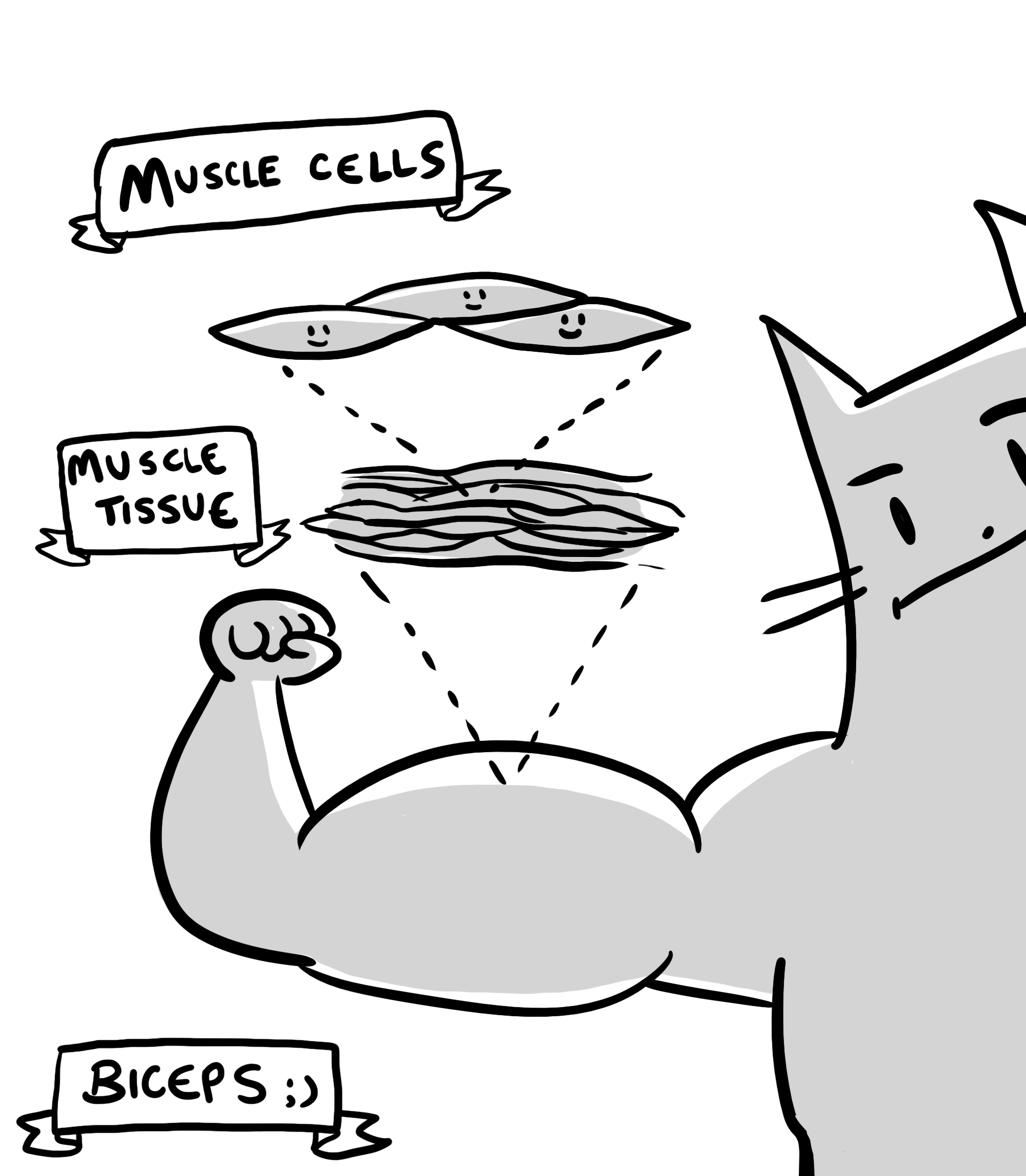
When forming complicated structures, cells are enclosed in a sort of three-dimensional fishing net, made of proteins, sugars and other molecules all released by the cells themselves. Think of it as a matrix of stuff that envelops the cells, which is fitting since this net-like material is known as the extracellular matrix. Incredibly, cells not only make this matrix, but they also organize it and destroy it in a highly regulated fashion. Furthermore, the extracellular matrix can also organize the cells themselves. It’s as if they are talking to each other, informing them of their surroundings.
How do the cells come together?
When cells do form tissues, they are essentially clumping together in a very organized manner. They can generally do this in one of two ways:
(1) a cell can directly hold onto a neighboring cell by way of a structures known as cell junctions, forming something known, not surprisingly, as a cell-cell junction; or
(2) a cell can interact with another cell indirectly, in a way where they are not really touching each other, but communicating to each other via this aforementioned extracellular matrix – we call this a cell–matrix junction.
This is partly why different tissues can physically be so different from each other. If you can imagine these differing ways of interaction, as well as consider the amount of cells compared to the volume of extracellular matrix, you can see how this is one way to introduce diversity into structures. For example, bones or tendons are made of connective tissue where the extracellular matrix is plentiful and cells are sparsely distributed within. Contrast that to tissues that make parts of your gut or your skin tissue, where cells generally adhere together into sheets.
In other words, the architecture, shape and strength of tissues depends a lot on the way cells come together, but beyond that, how cells function (i.e. what they can do) as well as how they keep healthy, will depend not on just how they “meet” but also in how they communicate with each other. Here, cells exchange signals that coordinate their actions. This tends to be through specific molecules belonging to one cell being sent and/or making contact with another cell. If cells can’t touch and communicate this can cause problems. They can’t talk properly, which in turn disrupt functions, and ultimately leads to disease. Just imagine the problems arising in a family where members decide to give each other the silent treatment, or worse start lying or yelling at each other.

Multiple examples show how diseases appear when there are problems in the way cells adhere or communicate. Bacteria, viruses or parasites often find ways to confuse these systems, since they must first adhere to host cells in order to infect and cause diseases. Another example is how cancer can spread throughout the body. Tumor cells tend to play it fast and loose with their adherence abilities allowing them to escape their site of origin and travel throughout the rest of the body.
Consequently, to fight many diseases, scientists need to understand the errors that occur when cells come together. This is a bit like acknowledging that to fix a car, it helps to know how the pieces of a car fit together.
Cell Adhesion Molecules, and introducing the Integrins
We know many details about how cell-cell junctions and cell-matrix junctions work. We know that cells use special proteins to interact and attach to neighbouring cells and/or the matrix. These proteins reside on the cell surface and are collectively known as cell-adhesion molecules (CAMs). Although there are many different types of CAMs, those know as integrins play an especially crucial role, because integrins are in charge of cell-matrix interactions.
Integrins span the cell membrane so that a part of the protein is outside the cell and another part is in the interior. The part outside the cell is the one that attaches to elements in the extracellular matrix, while the part inside the cell binds to the cell’s internal skeleton (this is called the cytoskeleton by the way).
If you think about it, this means that integrins link the exterior of the cell to the interior of a cell. In other words, these membrane spanning proteins are able send information out of the cell but also into the cell. In this way, the integrins can let a cell know what the outside situation is like (is there extracellular matrix out there, is it good, should we stick, should we let go)? Not only that, but the integrins help cells actually make decisions to react in certain ways (i.e. yes, I’m going to be extra sticky, or no, I’m want to be less sticky so I can let go).
In a nutshell, cells use their integrins to attach and detach to the extracellular matrix when needed, forming and breaking bonds. Scientists have actually found that integrins can change themselves between active and an inactive forms. This behaviour controls the way cells migrate inside our bodies when needed.
Integrins and disease
We, humans, have at least 24 types of integrins with different jobs in your cells. One way scientists understand the function of a cell’s component is to observe what happens if they remove it or get it to stop working. For example, they’ve observed that mice, who have had the gene for one type of integrin deleted, will develop weak muscles. This provides evidence that that specific integrin has an important role in producing healthy muscles.
Integrins are especially crucial in how your immune system works. Some of them are uniquely found in white blood cells and help them fight infection in the body. For instance, when white blood cells in charge of protecting the body against infections have reached the site of an attack, it makes sense that they become more sticky so that they can stay put in that area of infection. Furthermore, integrins not only help the white cells attach but they also mediate the processes require for the white blood cell to squeeze out of the blood vessel and directly into the damaged tissue area. People with problems in these type of integrins suffer from frequent bacterial infections. Other types of integrins are in the blood platelets cells that help the body form clots to stop bleeding, and people with problems in those integrins bleed excessively from wounds.
On the Shoulder of Giants
Dr. Timothy A. Springer, a professor from Harvard Medical School and Boston Children’s Hospital, dedicated his career to understanding the cell-cell interactions that happen in immune responses. He discovered the first family of integrins and figured out how integrins were able to send information out of and into the cell. His discoveries allowed the scientific community to understand how a faulty integrin can cause disease. Just as important, he led some of the first research that demonstrated how this knowledge can be used to help fight disease. Thanks to this remarkable contribution, Dr. Springer is the recipient of the 2019 Canada Gairdner Award, which recognizes world-renowned scientists for the impact of their research to human health.
Hopefully, you now know a bit more about how cells form communities, touch, talk, send messages and how we get sick if they fail at these tasks. Central in this scientific story are the class of cell adhesion molecules known as integrins. Just another example of the amazing abilities of your cells – definitely not just squishy bubbles of fluid.
Learn more about UBC’s Michael Smith Laboratories, the Gairdner Foundation, and the Canadian Society for Molecular Biosciences.
Discussion Questions For High School Teachers and their Students
For all four articles:
The materials presented here represent authentic research that is probably pretty specific (i.e. not directly covered in your high school course work). What parts of your curriculum (your course outline) are related to the topics written about in each of the four papers (or choose one)?
For each of the four topics presented (or choose one), how would build a model using recycled materials to illustrate the key concepts of the topic?
These materials describe scientific discoveries that can benefit human medicine. However, many discoveries don’t necessarily start off with the intent of being used in medicine. Research for the sake of curiosity and fundamental obtainment of knowledge (i.e no initial applied outcome) is often referred to as basic research. Why do you think basic research is so important for science and society?
For “Kinesin: The Little Engine that Could”:
The paper mentioned that a cell has a sort of “skeleton” of its own, called a cytoskeleton. The paper also mentioned a few reasons why this is important – can you highlight them? Can you think of other reasons why a cytoskeleton might be important?
The fact that Dr. Vale discovered kinesin using nerve cells from giant squids might sound strange, but in biology, it’s not uncommon to make discoveries by studying other organisms. Why do you think this is the case? Look up the term model organism, and discuss an example of a model organism.
For “DNA Replication: Not your Office Photocopier”:
What would happen to your cells if they were only able to initiate replication at one site of each cell’s genome? What would happen to you?
Cancer often requires the accumulation of multiple mutations in the DNA (i.e. lots of things need to go wrong for a cancer effect to be seen). As outlined in the paper, often one of the first mutations involves one that makes DNA replication itself more error prone. Why might this lead to a cascading effect, and ultimately lead to the cancer itself?
For “Plants: Amazing Phyto-Pharmacies”:
Phytochemicals are often the reason why many herbal remedies work. But how is a medicine in the form of a phytochemical different from that of an herbal remedy?
Do a little research on the term biopiracy. What do you think of this issue?
For “Integrins and the Social Network of Cells”:
If you’ve had an infection before, what are some of the symptoms that you are familiar with? Do any of these seem to relate directly to how integrins might be involved?
The paper made mention of cancer. Look up the word metastasis, and discuss in the context of how a defective integrin protein might contribute.
Download printable documents
Download all resources available:
Download the zines:
Download the comics:
Download the articles separately:
Taxol – Horwitz Kinesin – Vale DNA Replicaton – Stillman Integrins – Springer
Download the discussion questions:
Thank you to all the writers and artists who made these articles possible. We invite you to view and share these resources widely, as they highlight the impact science has on our lives and our understanding of the world.