Awards and recognition
The Director’s Kickstart Award scores new collaborations within the Michael Smith Laboratories
Written by MSL Postdoc Communications Team including Julie Grondin, Laleh Solhi, and Alexandra Sébastien.
Interdisciplinary collaborations can lead to new ideas and better solutions. Last year, the Michael Smith Laboratories established the Director’s Kickstart Award to support new research collaborations, projects, and directions within the Michael Smith Laboratories. The goal of this funding was to advance research and support new innovative collaborative initiatives to generate preliminary data for future grant applications to external funding agencies. Last year’s competition created a lot of positive energy and excitement. On October 17th 2018, Dr. Peter Zandstra, Director of the Michael Smith Laboratories, hosted the “Kickstart Project Presentations” where last year’s award recipients shared a short presentation about their projects journeys and outcomes.
“Sometimes, researchers only stay within the limits of their own discipline,” said Dr. Zandstra. “These awards give members of the Michael Smith Laboratories an opportunity to collaborate on projects with other labs in the unit resulting in expertise and resources they might not have had access to without this award.” Dr. Zandstra added, “It also gives them a chance to test preliminary data, using interdisciplinary methods, in which they could use for future grant applications to external funding agencies.”
Learn about the 2018 award recipients and their projects:
 Mélissa Caza and Guillaume Déjean (Kronstad & Brumer labs)
Mélissa Caza and Guillaume Déjean (Kronstad & Brumer labs)
Title: Hunting for microbial enzymes that target the capsular polysaccharides of the human pathogens Cryptococcus neoformans and Cryptococcus gattii
Mélissa and Guillaume aimed to identify microbial enzymes that can degrade Cryptococcus capsular polysaccharides which could lead to enzymatic therapy and vaccine development as well as providing new tools to investigate the structure-function of the capsule. With an unusual angle of ‘’breaking down their sugar coat”, they explored a new therapeutic avenue for the human disease cryptococcosis caused by Cryptococcus spp. More specifically, they want to discover enzymes that could target the polysaccharides from the fungus cell surface, which would leave the fungus unprotected and vulnerable. This is significant because cryptococcosis affects both immunocompromised and immunocompetent people. The global burden of cryptococcosis is evaluated at nearly 200,000 death per year. Unfortunately, a very limited set of antifungal drugs are available.
Thanks to the MSL Kickstart Award, Mélissa and Guillaume hired a co-op student, Andrew Wilson, to help with the project. This opportunity provided mentorship and training for Andrew and created a unique occasion for them to work together and learn about each other’s complementary speciality, respectively fungal pathogenesis (Kronstad lab) and carbohydrate enzymology (Brumer lab). After one year, their experimental findings were a bit unexpected. They found a soil bacterium Pseudomonas fluorescens that can grow on the capsular material but reduces the viability of the fungus. This turn of events led them to seek a third partner in this adventure, the Haney lab, which specialized in plant bacteria. This project is a great example of collaborative work and an ever-evolving project which improves with each new discovery. Mélissa and Guillaume are excited about their recent findings and hope to continue their collaborative work.
 Jörg Gsponer and Thibault Mayor (Gsponer & Mayor labs)
Jörg Gsponer and Thibault Mayor (Gsponer & Mayor labs)
Title: Proteome-wide identification and analysis of protein autoinhibition
Jörg and Thibault led an exciting Kickstart project focusing on the discovery and identification of key autoinhibition proteins. When asked to describe their project to a child, Thibault explained, “Some proteins, which are tiny elements of our bodies, work like a light switch. In some diseases, these light switches are turning on by themselves. We are developing a computer program to predict which diseases are caused by defective light switches.” Jörg and Thibault are currently collecting more information to prove whether this process is actually happening within the human body.
The MSL Kickstart Award gave them a chance to “crash-test” new ideas and begin their long journey towards the discovery of new treatments for various rare genetic diseases. This collaborative project was an opportunity to use their complementary methodologies, which are computational biology for Jörg and proteomics for Thibault, to expand on their current research and to develop new ways to identify solutions. Throughout this project, both labs benefited from the Kickstart Award as they were able to learn about each other’s methodology which has led them closer to identifying the “switches”. Not only did this Kickstart award connect the two labs through research, but it also gave Jörg and Thibault a chance to connect and discuss science, and their “common love for mountains, cheese and wine!”
 René Pedroza and Eleonor Rayner (Piret & Kastrup labs)
René Pedroza and Eleonor Rayner (Piret & Kastrup labs)
Title: Novel bioengineering materials and device concepts to facilitate development of encapsulation systems for Type 1 Diabetes
René and Eleonor collaborated on a Kickstart project to test whether platelet-rich plasma (PRP) could improve engraftment of alginate-microencapsulated islets transplanted intraperitoneally by preventing microcapsule sedimentation seen in humans. Proof of principle for this idea was obtained by showing that clotted PRP adhered alginate microcapsules to excised murine peritoneal tissue. When injected intraperitoneally in mice, syngeneic PRP did not cause any adverse effects in vivo over 8 weeks of implantation. Further tests aimed to develop a method to show that PRP can prevent sedimentation of alginate microcapsules in situ by maintaining them adhered to the peritoneal walls of euthanized mice. However, the method developed was not sufficient to induce sedimentation in euthanized mice neither in our control mice nor those that received PRP.
Secondary outcomes of this collaboration include the development of several methods for controlling the initial intraperitoneal distribution of alginate microcapsules, and a method for visualizing microcapsules implanted intraperitoneally in euthanized mice. Eleonor, a Lab Manager for the Kastrup Lab, added, “these will be useful for future experiments, testing whether PRP can maintain alginate microcapsules adhered to the peritoneal walls of live mice.”
The method to mimic sedimentation seen in bipeds, or movement of the microcapsules from the site of implantation, needs to be refined. Once sedimentation can be replicated in euthanized mice, the method can be adapted for use in live mice. René, a Ph.D. student from the Piret Lab states, “the ultimate aim will be to test whether alginate microcapsules containing Islet cells can be adhered to the peritoneal wall in such a way that the distribution is maintained in vivo until tissue healing processes are able to grow a vascular network for exchange of nutrients and signalling proteins with the cells.” The pair said they would like to use the preliminary data produced from the Kickstart award to apply for larger funding opportunities.
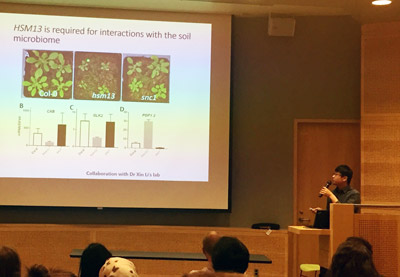 Yi Song and Xin Li (Haney & Li labs)
Yi Song and Xin Li (Haney & Li labs)
Title: Elucidating the role of plant immunity in shaping rhizosphere commensals
With the help of the MSL Kickstart Award, Dr. Yi Song, a post-doctoral fellow in the Haney lab, and Dr. Xin Li, an expert on plant immunity, set out to identify how plant immunity components regulate the beneficial root microbe levels. Yi identified a novel auto-immune mutant of Arabidopsis which showed significant growth defects compared to healthy plants. He also found that the rhizosphere of this mutant was enriched in the commensal rhizosphere bacterium Pseudomonas fluorescens. Subsequent experiments lead Yi discovered that this mutant used a specialized signalling system to regulate the levels of beneficial microbes in the rhizosphere. “This means that plants can call for help, and they can recruit beneficial microbes to enhance their resistance to further stresses.” Yi will use microbiome 16S-based community sequencing to assess the overall change in the composition of the rhizosphere and to identify the role that plant immunity plays in regulating this complex ecosystem.
Received the Kickstart Award enhanced Yi’s exceptional research record, which supported his Banting Postdoctoral Fellowship application to be chosen as a UBC Nominee and has now moved forward for consideration at the national level. Aside from the ability to leverage the Kickstart Award for fellowship funding, this award directly supports Yi’s long-term research interests. “I am particularly interested in interactions between plants and the whole microbiome. This project provides funding to do root transcriptomic analysis and 16S community sequencing during interactions with beneficial microbes.”
James McCoy, Jennifer Bui and David Rattray (Finlay, Gsponer & Foster labs)
Title: Revealing the Substrates of Secreted Enteropathogenic E. coli Kinases
During infection, the human gut pathogen Enteropathogenic E. coli (EPEC) secretes molecules into the host cells including several proteins called effector kinases. These modify host signaling proteins by phosphorylation, and can alter their function. A key step to understanding the mechanisms of EPEC infection is to identify these host proteins, however, pinpointing kinase targets is notoriously difficult. “The project at its core is trying to develop a technique to study the activity of kinases…and to do that in cell culture rather than in cell lysate or in vitro like we usually do,” explained James McCoy, a post-doctoral fellow in the Finlay Lab. Together, with research associate Dr. Jennifer Bui from the Gsponer Lab and PhD student David Rattray from the Foster Lab, the three set out to overcome this challenge using computational modeling and protein engineering.
The team modified a method called “substrate capture”. Jennifer used computational modeling to engineer mutants of the EPEC kinases that would be able to recognize modified substrates displaying custom chemical tags. Next, James performed live culture assays to confirm that these modified kinases produced normal infections, and to measure the activity of these mutants in vivo. To date, the team has identified a promising kinase mutant and has used microarrays to identify its candidate host protein targets. Moving forward, the exact site of the phosphorylation introduced by the EPEC kinases to these host proteins will be identified by David using mass spectrometry.
“The Kickstart Award gives us hope that we can establish the protocol for the design of these kinases,” said Jennifer. It also provides expanded training opportunities for students like David. “I’m always looking for a chance to do more bacterial work since my PhD revolves around host-pathogen interactions, so the Kickstart [Award] has been really good for putting me on a different project…and to expand the scope of my research.”
Dave Ng and Cara Haney (Ng & Haney labs)
Title: Whole genome sequencing of Pseudomonas fluorescens strains. A high school fieldtrip producing relevant scientific results
“Because this experiment is pretty high tech and generates real data, I think that the authenticity [of being a part of a real research project] can actually have strong impact for the kids who come through the program,” Dave Ng.
The Michael Smith Laboratories, whilst well known for its research chops, is also a place where community, outreach, and learning can come together. An example of this is a new educational initiative about to start in 2019. Here, Professors Dave Ng and Cara Haney, are collaborating on a Kickstart project where they plan to host a new field trip program for high school students to sequence the genomes of beneficial Pseudomonas fluorescens bacterial strains. Furthermore, the data obtained by the teenagers would later be used for full research analysis in the Haney lab, making the experience an authentic opportunity to do “real” science (in this case, using next generation sequencing methodologies). Dave is the Director of the Advanced Molecular Biology Laboratory which is the educational outreach component of the Michael Smith Laboratories, and Cara leads a lab that specializes in plant-microbiome interactions.
Developing the field trip required sensitivity to both STEM learning outcomes as well as recognition that the practicality of the experiment may meet a number of challenges when performed by inexperienced hands. Still, many of these technical and logistic issues have been worked through with the help of some great high school students, brave enough to beta test the project during the summer and fall of 2018. In the end, Dave and Cara hope that this type of opportunity will both have a lasting impact on the youth who participate, and also via the insights obtained from the data itself.