Newsroom
Honing the immune cell production pipeline: How Zandstra lab researchers are advancing cellular therapy
Great gains have been made in the field of stem cell research, especially in the last five years, using immune cells to treat cancer. Despite the progress, the bottleneck of costly and laborious means of production for personalized medicine has remained a barrier. Dr. Yale Michaels and researchers from the Zandstra lab at UBC’s Michael Smith Laboratories and School of Biomedical Engineering have published a breakthrough paper in Science Advances that just might change the game. The research team has refined a process that produces cancer fighting immune cells from pluripotent stem cells faster, and more efficiently than previously known methods. With a patent application in the works, this exciting finding could help transform the field of stem cell medicine from an expensive, niche endeavour to something easily scalable and broadly applicable in the clinic.
CAR T therapy, a well-known and successful cancer treatment, involves obtaining immune cells from the patient, genetically modifying them to fight against the patient’s cancer and infusing them back into the patient’s body to fight the disease. Although this type of therapy has an efficacy rate of close to 50% for some cancers, a new batch of medicine needs to be created for each treatment, costing roughly half a million dollars each round.
“Because the main cost associated with these treatments is the fact that they’re made individually, a more cost-effective strategy could be figuring out how to manufacture those immune cells in the lab using stem cells, instead of taking them directly from a patient,” explains Michaels.
Building on a large body of previous work in the area, Michaels and team have discovered an efficient way to do exactly that.
“We’ve figured out the minimal necessary steps to efficiently guide pluripotent stem cells to develop in the dish into immune cells, in particular, T cells.”
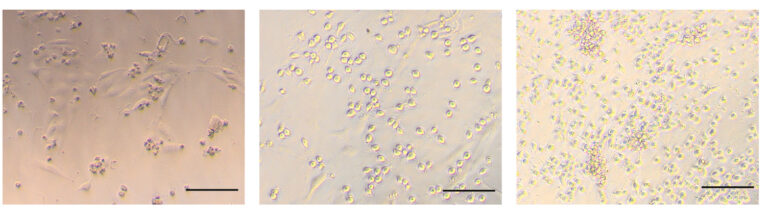
T cells, important infection fighters in our body, are derived from blood cells, which originate from pluripotent stem cells (PSCs). This developmental pipeline has been difficult to generate effectively in the lab, until now. Michaels and his team discovered that by providing two proteins to the cells during a key window of the development improved the efficiency of immune cell production by eighty times. Their novel method is the fastest known means of production of immune cells in the lab. Additionally, their method removed the use of animal cells and serums used in previous methods that complicated the process and varied results. These previously used feeder tissues and serums aren’t chemically consistent and harbour the potential for infection in the patient. By working strictly with these two proteins instead, DLL4 and VCAM1, the production process becomes a carefully controlled pipeline that is easy to replicate.
Pluripotent stem cells are powerful tools for cellular medicine. They have the ability to differentiate into any type of cell in the human body and they can endlessly renew themselves. Using PSCs to create immune cells in the lab for therapeutic treatments means hundreds of doses of a medicine could be derived from a single cell.
“One of the next steps we’re working on is to scale this up and make it work more efficiently so that we can make enough cells to treat patients. I hope that this publication and the ongoing work in the lab will contribute to future clinical pipelines,” says Michaels.
The improvement of this production pipeline is one step among many towards solving a variety of human health challenges. How to scale up a cell differentiation process, how to make cells good at killing cancer and fighting against other immune diseases and how to deliver them to patients in a safe way are all important questions being explored simultaneously by other research groups. It takes large scale collaboration to solve these types of challenges. The success of this research project within the Zandstra lab is the culmination of effort from a diverse team of people; engineers, molecular biologists, computer scientist and so on.
“It’s been quite rewarding seeing all the fruits of those different knowledge bases coming together,” Michaels reflects.
And the decades of previous stem cell research and inroads made in the last five years laid the groundwork for this breakthrough.
“It’s the collective work of hundreds or thousands of people, each making important contributions that collectively enable this thing to succeed. People have made tremendous progress over the last 20 years; this breakthrough is an exciting continuum.”
Personalized medicine and stem cell research is a burgeoning field that requires patience and constant curiosity. And seeing the potential for findings like these to work towards treating human problems like cancer, immune diseases and transplant rejection is hugely motivating for Michaels.
“It’s fascinating to think that one single cell can produce all the cells, with their diverse functions, required to make up an adult human body. And how that happens is an interesting fundamental question about what makes us us. The more we learn about how this process unfolds, the better we are able to apply it to treating human disease.”
We look forward excitedly to where this research will lead the field next.
Quick links:
- Read the publication:
DLL4 and VCAM1 enhance the emergence of T cell–competent hematopoietic progenitors from human pluripotent stem cells - Learn more about the Zandstra lab